
The experiments use human cells to imitate the blastocyst phase — offering a crucial window into human development.
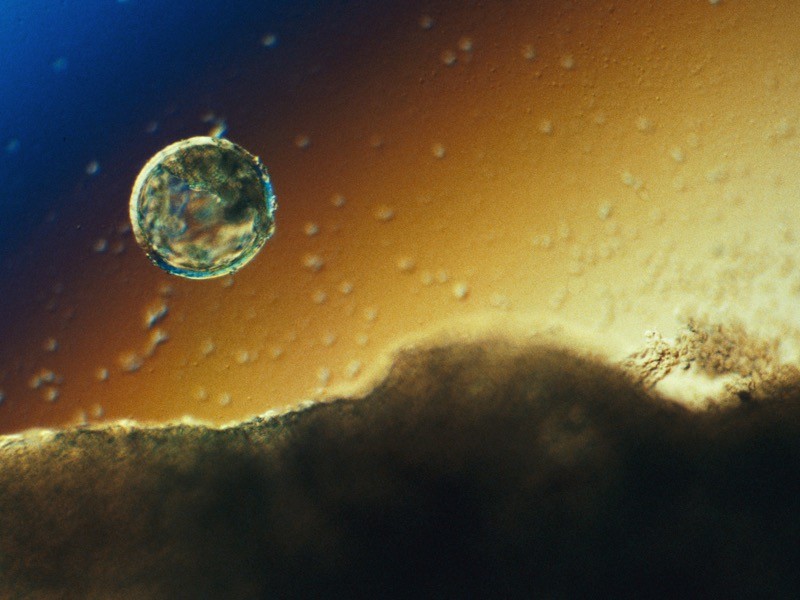
A blastocyst will implant in the wall of the uterus around day 7 or 8 of human development.Credit: Lennart Nilsson, TT/SPL
Some knowledge of this phase comes from research groups growing human embryos in the lab for up to 13 days. Laws in about a dozen countries, as well as guidelines issued by the International Society for Stem Cell Research (ISSCR), limit embryo development in the lab to 14 days after fertilization. By this time, implantation is complete, and what’s known as a primitive streak appears in the embryo, marking a point at which the cells within are becoming more differentiated and complex.
In one of the two Nature studies1, a team of scientists at the University of Texas Southwestern Medical Center in Dallas and Kunming Medical University in China treated human stem cells with a succession of growth factors to form artificial blastocysts, called ‘human blastoids’. The researchers showed they could do this using stem cells derived from human embryos, or using adult skin cells that had been reprogrammed into stem cells.
In the other peer-reviewed study2, Jose Polo at Monash University in Clayton, Australia, and his colleagues reprogrammed adult skin cells to generate a mixture of cells, some of which grew into human blastoids.
"“Is [a human blastoid] exactly equivalent to a human embryo?” asks Aryeh Warmflash, a stem-cell biologist at Rice University in Houston, Texas. "Almost certainly not. Is it a pretty good model for the blastocyst-stage embryo? I think it probably is.”
Both teams showed that their artificial structures were built like blastocysts, with a cavity in the centre and a mass of cells — what could continue developing into fetal tissues in real blastocysts — in one corner. They also showed that the structures contained three signature cell types that make up a blastocyst. And they coaxed their human blastoids to ‘implant’ onto plastic sheets and mature into a state similar to a human blastocyst after it implants into the uterine wall.
The protocols are familiar to researchers such as Fu, who has seen similar methods used to develop mouse blastoids. “Nonetheless, this is a very important next step,” he says.
The two teams that posted preprints showed similar results while working with extended pluripotent stem cells.
“Looking to the future we want to use this model to gain more insight into early human development, and to understand different gene functions, as well as their mutations,” says Jun Wu, a molecular biologist at Southwestern, who led one of the Nature studies1.
Still, the teams acknowledge that their methods can be improved. Both Nature studies reported that only about 10% of the reprogrammed or transformed cells developed into human blastoids. Also, both teams acknowledged that there were some cells in the structures that are not typically found in human blastocysts.
“It’s a good start,” Rossant says of the studies. But based on these factors, “you would predict that it’s not going to be incredibly reproducible”.
Neither of the teams that published in Nature allowed their structures to grow beyond about the equivalent of a 2-week-old embryo, mindful of the 14-day rule's limit on growing human embryos in the laboratory.
Still, some developmental biologists think that these artificial structures differ from human embryos in a key way. Scientists do not expect the structures will be viable beyond this stage of development, based on evidence7 showing that mouse blastoids do not develop into embryos when implanted in a mouse uterus.
But their similarity to human blastocysts still raises ethical questions. The ISSCR is already aware of this, and is due to release revised guidelines for work with embryo-like structures in May.
The sophistication of these model structures and the uncertainty about their developmental potential and whether they should be treated as embryos has made it hard to get funding for the work. In the United States, the National Institutes of Health has been reluctant to fund such work, citing a section of federal law known as the Dickey–Wicker Amendment, which bars the government from funding research that creates or destroys human embryos. Researchers argue that the structures are different from natural human embryos and have called for clarity from the agency on the criteria that guide its funding decisions.
The agency’s policy office last year convened a meeting of leading researchers at the US National Academies of Sciences, Engineering, and Medicine in Washington DC to discuss milestones in the field. This month, NIH director of science policy, Carrie Wolinetz, wrote that the agency would consider funding stem-cell-based model structures that mimic embryo development on a case-by-case basis.
